The energy sources of the future come in the shape of gas: methane, biomethane and hydrogen. Gas is future fit. It is low-emission, innovative and supply-secure.
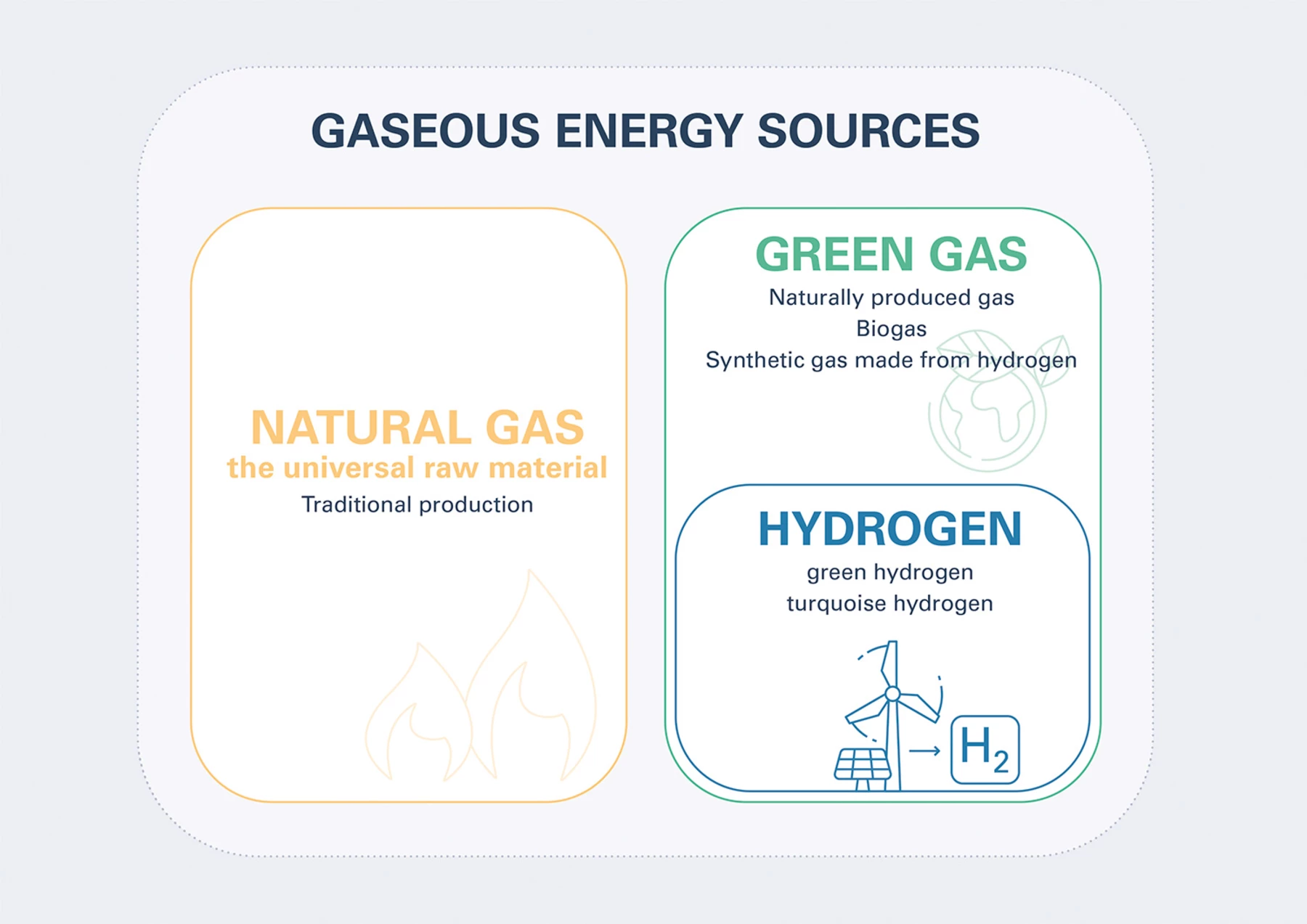
The range of gaseous energy sources is broad
The spectrum of gaseous energy sources runs from conventional natural gas to green gas such as hydrogen and biomethane. Gas underpins the attainment of climate goals, is a partner for renewables and can even be produced renewably from wind and solar energy or biomass.
Energy in the form of gas can be transported out of sight underground, stored in huge quantities, made available quickly in large volumes, and used in a variety of applications to generate electricity and heat.
 ©
©
Natural gas – the universal raw material
Natural gas is a raw material with universal applications. Natural gas deposits are principally composed of methane (CH4) – a simple compound of carbon (C) und hydrogen (H), which can be bonded and separated synthetically. Natural gas has a pivotal part to play in the progressive roll-out of a sustainable energy system, and will help us along the road to a renewable energy future.
 ©
©
Green gas – standard bearer for the future of energy
Green gas stands for all gaseous energy forms that offer carbon free, low carbon or carbon neutral production and consumption. Whether in the shape of hydrogen produced by means of water or methane electrolysis, biomethane, or naturally produced green gas from our Underground Sun Conversion project – gaseous energy sources are the future. Green gas not only has vast potential, it is sustainable, affordable and storable. It is paving the way for a sustainable energy future.
Naturally produced synthetic gas
First, hydrogen (H2) is produced using solar or wind energy in a process known as power-to-gas. The hydrogen can be directly injected into RAG’s storage facilities, to be withdrawn as needed. Or, in a second step, carbon dioxide (CO2) can be added, and the hydrogen is converted into naturally produced natural gas (CH4) underground, where it can be stored in large volumes to meet seasonal demand peaks. This creates a sustainable carbon cycle. The green gas produced in this way is carbon neutral.
Synthetic gas
Synthetic gas means gas that is produced by means of electrolysis or splitting, using green electricity and an abundant feedstock (water or methane). It includes green and turquoise hydrogen, as well as synthetic natural gas produced from hydrogen in an underground methanation process (Underground Sun Conversion project).
 ©
©
Clean hydrogen
Green hydrogen is produced by water electrolysis, exclusively using electricity generated from renewable sources.
Water electrolysis: H2O + 1 energy = H2 + O
Turquoise hydrogen is produced by electrical splitting of natural gas or biogas (methane electrolysis), where methane is broken down into hydrogen and solid carbon in an emission free process. This means that carbon, a valuable raw material, can be obtained at the same time as storable, climate neutral hydrogen, using just a quarter of the renewable energy required by water electrolysis.
Methane splitting: CH4 + ¼ energy = C + 2 H2
